Description
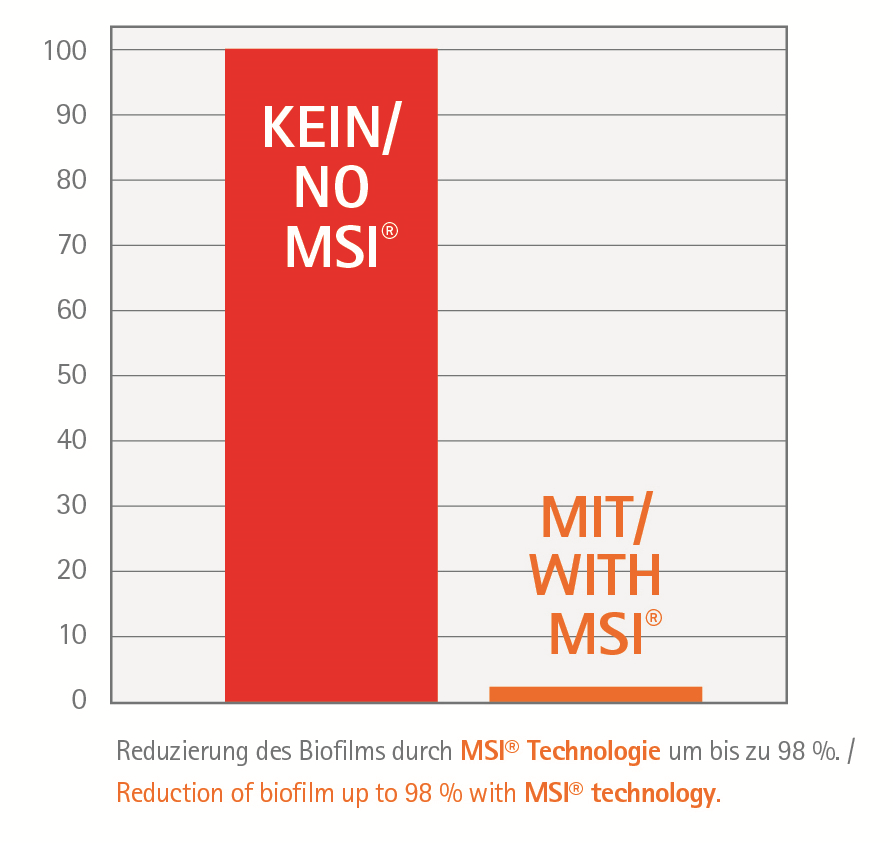
Pro3dure´s 3D printing resins of the printodent® GR-10 series are biocompatible resins for the fabrication of splints and implant drill guides. In addition to its exceptional elongation for this class of material, printodent® GR-10 guide is also characterized by its high transparency and UV stability. This makes it easy to insert sleeves into the guide and gives you optimum control during the operation. The material is now also available in the printodent® GR-10 guide | MSI version as a functional 3D printing resin with MSI® technology, which significantly reduces biofilm and plaque build-up on surfaces. MSI® is based on the patented mode of action of special natural compounds that disrupt the communication of bacteria (quorum sensing). Inspired by these natural compounds, which are produced by the red alga Delisea, a sustainable approach to reduction biofilms has been found that, unlike antibiotics, for example, does not cause bacterial resistance.
Advantages:
- outstanding mechanical properties
- reduced biofilm adhesion by MSI® technology
- high UV stability
- sterilizable
Available in ocean-blue color for 3D printers that emit light at ≤ 405 nm.
COMPATIBLE WITH DLP/LCD/SLA 3D PRINTERS.
Specifications for Pro3dure printodent® GR-10 guide | MSI
| Property | Requirement | Result*** | Standard |
| Flexural strength | ≥ 50 MPa | 64 MPa** | ISO 20795-2 |
| Flexural modulus | ≥ 1500 MPa | 1584 MPa** | ISO 20795-2 |
| Water solubility | ≤ 5 µg/mm³ | 1,4 µg/mm³ | ISO 20795-2 |
| Water absorption | ≤ 35 µg/mm³ | 24 µg/mm³ | ISO 20795-2 |
| Shore Hardness | n.a./n.a.* | 80 D | ISO 48-4 |
| Viscosity (23°C) | n.a./n.a.* | 0.7 Pa s | DIN 53019-1 |
| Maximum stress intensity factor | ≥ 1.1 J/m^2 | n.a./n.a.* | ISO 20795-2 |
| Total fracture work | ≥ 250 MPa*m^1/2 | n.a./n.a.* | ISO 20795-2 |
| Bio compatibility | Irritation and delayed-type allergies | Comply |
ISO 10993-1 ISO 10993-10 |
| Bio compatibility | Genotoxicity, Carcinogenity and Reproductive toxicity | Comply |
ISO 10993-1 ISO 10993-3 |
| Bio compatibility | Systemic toxicity | Comply |
ISO 10993-1 ISO 10993-11 |
| Bio compatibility | Cytotoxicity | Comply |
ISO 10993-1 ISO 10993-5 |
* not applicable
** on the basis ISO 20795-2
*** These data result from measurements of a representative sample, which were determined within the scope of our quality assurance
How to use
Preparation:
-
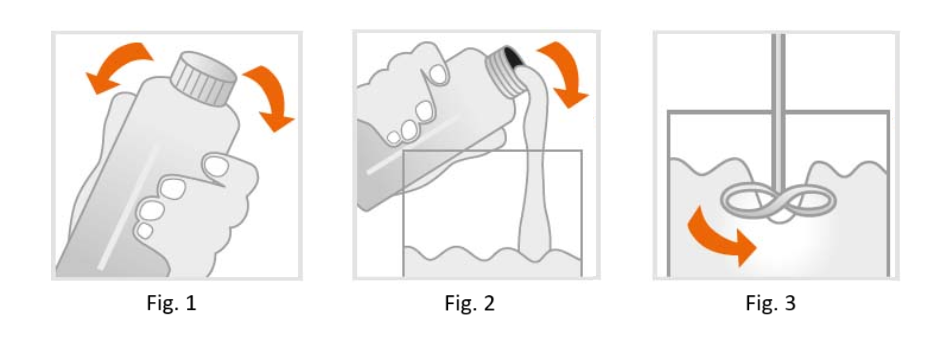
GR-10 guide | MSI bottles should be well shaken before use (Fig.1).
-
Make sure that GR-10 guide | MSI temperature is within 23 °C to 30 °C. If possible, always store a bottle GR-10 guide | MSI in your production unit in order to avoid temperature differences during refilling.
-
Impurities due to operational mistakes cannot be excluded. With respect to the low viscosity of the resin it is possible to filtrate GR-10 guide | MSI and stir it on a regular base (Fig.3). To avoid bubbles let GR-10 guide | MSI rest for 1 hour before usage.
Manufacturing process:
- Prepare data (CAD & build preparation).
- Choose process parameters (build style, etc.).
- Transfer prepared data to 3D printing device.
- Prepare 3D printing – shake bottle.
- Fill resin tank of 3D printing device (Fig.2). Bubbles can be removed with a clean spatula or by a recoater routine.
- Build the parts.
- Clean parts (with IPA ≥ 97 % or equivalent cleaning agent)
approx. 10 min. in an ultrasonic bath or equivalent
device – precleaning recommended. - Dry parts, until there are no residues of IPA or equivalent
cleaning agent. - Post curing (2 x 5 min. / wall thickness > 2.5 mm: 2 x 10 min.):
inert atmosphere recommended (use adequate light
curing device). - Finish parts. The dental objects created with GR-10 guide | MSI can be coated and repaired as usual.
Important
-
To avoid detrimental effects on material quality do not expose the liquid material to irradiation under any circumstances.
-
Deviations from the described manufacturing process may lead to different mechanical and optical properties of the GR-10 guide MSI material.
-
Ensure personal protective gear during processing.
-
Caution: Polymerized resins are chemically resistant – avoid stains on clothing!
-
Avoid any contact with skin and eyes. In case of accidental contact, rinse with adequate running water, consulting a doctor if necessary.
-
The lot number and the best before date are indicated on each GR-10 guide MSI packaging. In case of claims please always indicate the lot number of the product.
-
Do not use the product after expiry of the best before date.
Safety notice
The company I.D.M di Marin Davide is not a manufacturer of medical materials or devices. The material sold on this site is neither produced nor controlled by the company I.D.M. di Marin Davide, who in no case can be held responsible for any damage caused by use or application of the material, which must only be used on suitable machines and by specialized personnel, exclusively for the purpose indicated, according to the manufacturer’s instructions. For resins with European certification, the properties of the finished product vary according to the entire printing and post-curing process; refer to the manufacturer for any information about it.





Reviews
There are no reviews yet.